Decoding the bull: Your step-by-step guide to semi-automated morphology and morphometry assessment of bull spermatozoa
Did you know that the morphology of spermatozoa can reveal both the species it belongs to and its evolutionary relationships? In the bull breeding industry, sperm morphology is used as one of the semen quality measurements and can be a reason for rejecting an ejaculate for artificial insemination AI dose production. Sperm morphology analysis is also an important part of the Bull Breeding Soundness Examination (BSE), which aims to identify sub-fertile bulls in herds under field conditions.
Many studies have shown that subjective sperm morphology assessment is imprecise and lacks repeatability due to differences in classification and stains used, human error, and technician variability. To achieve more objective and standardized results, Computer-Aided Sperm Morphology Analysis (CASMA) can be used. The CASMA method combines brightfield microscopy with software that detects sperm components from smears of stained sperm. We refer to our method as “semi-automated” because it requires manual addition of some of the specie specific abnormalities and tail defects.
Below is a step-by-step guide to semi-automated morphology assessment of young Norwegian Red bull spermatozoa, which can be applied to other breeds and species.
Step 1: Do Your Background Check! – Understanding Sperm Morphology for Better Analysis
Bull morphology and morphometry is well-researched, including information on abnormality classification, comparison of sperm stains and use of new statistical methods for morphometry clustering.
Understanding the classification of morphology abnormalities and their etiology will allow you to have a better idea of the impact they have on the production and quality of the AI doses and consequently fertility of the bull. Sperm abnormalities can be divided into those with compensable traits – sperm that cannot reach the oocyte but with an increased number of sperm per insemination dose, we can improve the fertilisation rate – or uncompensable traits – sperm that can fertilise the oocyte but the abnormality causes embryonic death or abnormal development.
There are no global standardised thresholds and classification for the morphological abnormalities of bulls, but there is range of frameworks to choose from such as one made by Nöthling (2008). I used a standardised method for BSE in Australia, described by Perry (2021).
Step 2: Choose and Adjust Your Staining Protocol for Accurate Assessment
The next step is to choose a staining method most suitable for your species and a stain which allows for the differentiation of individual sperm components (e.g.head, acrosome, midpiece and tail) . Based on my previous experiences, I selected SpermBlue which was successfully used in a range of species in combination with CASMA systems. SpermBlue fixative and stain are iso-osmotic and isotonic in relation to the semen which results in less distortion of the sperm head dimensions, as was found for other stains.
Always take time to test and adjust your staining protocol – good staining (Figure 1) will save you a lot of time during the process of capturing images with the CASMA system. Please note that your laboratory environment, type, and quality of distilled water can influence your staining and washing time. Figure 2 shows my adjusted SpermBlue staining protocol for Norwegian Red bulls’ spermatozoa.
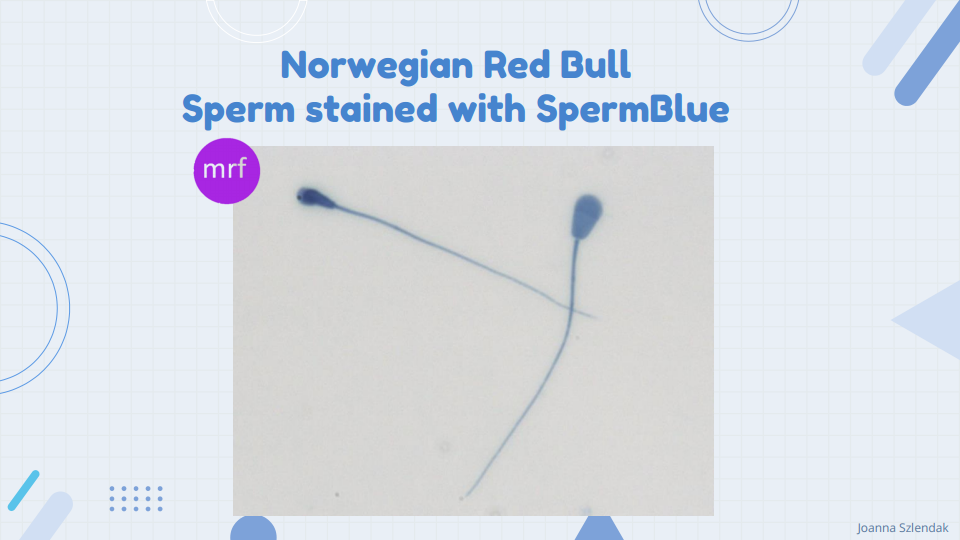
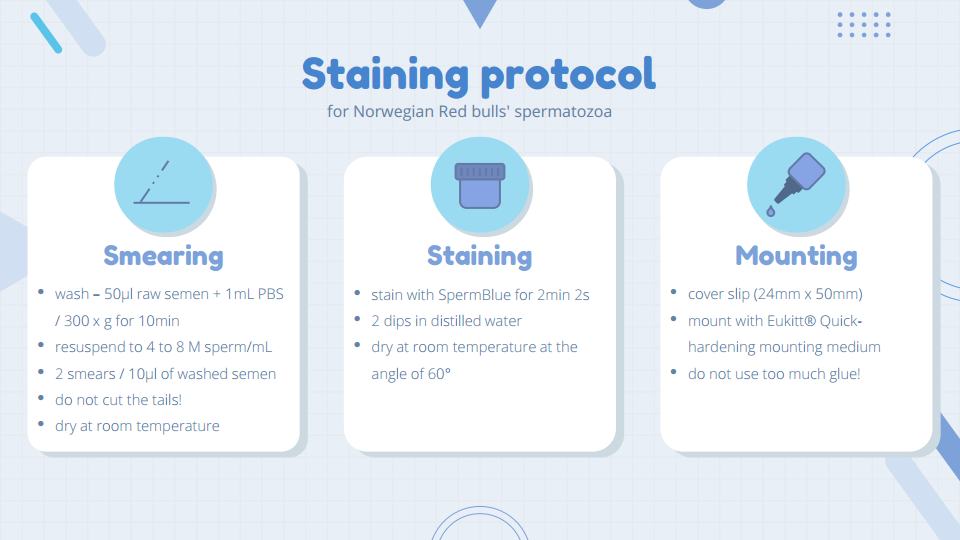
Remember to always make multiple slides per ejaculate – this will ensure that you have enough sperm cells to capture and will save you time in case of any emergency (believe it or not, glass slides sometimes fall and break).
Step 3: Capture Spermatozoa with CASMA – Ensuring Sufficient High-Quality Data for Analysis
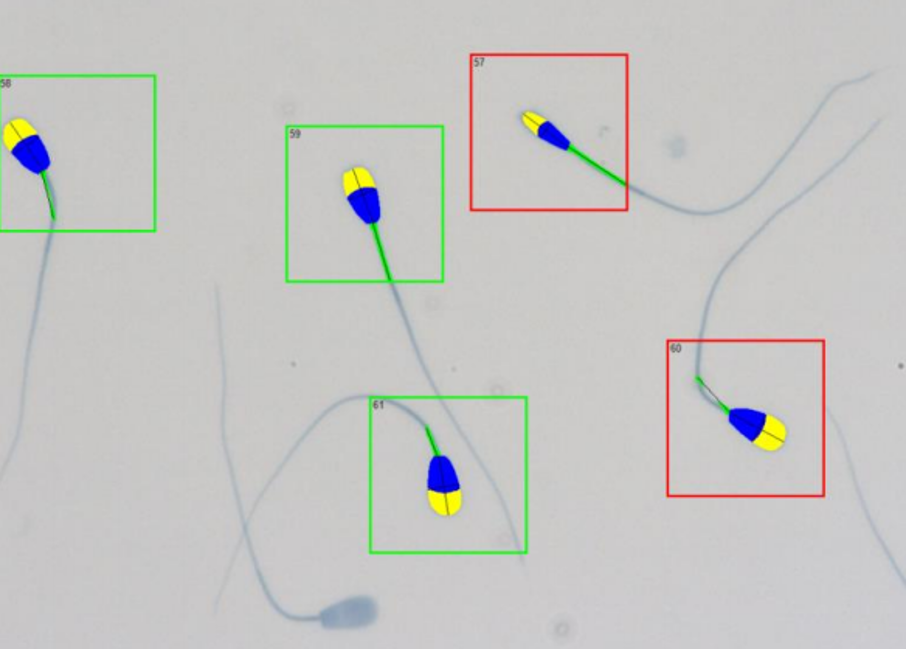
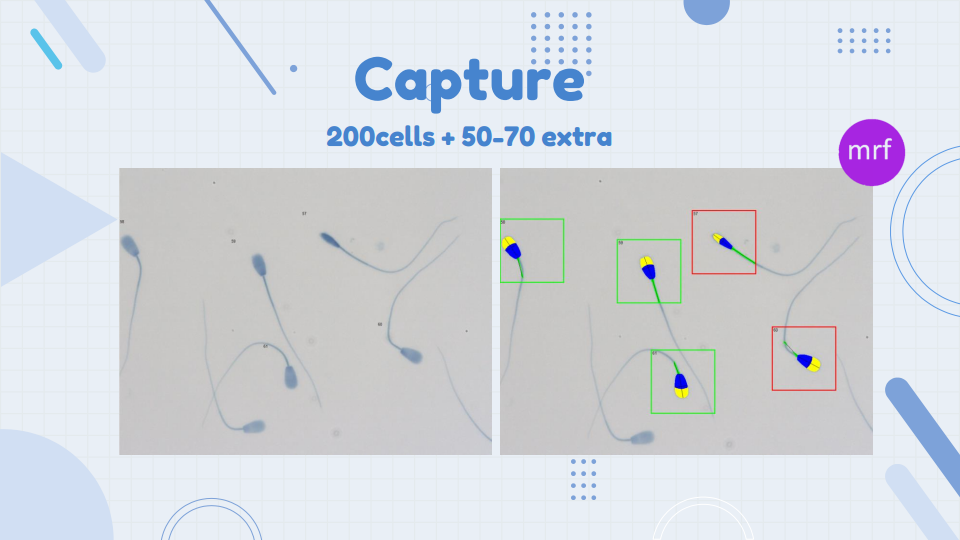
After staining and mounting the slides, assessment of the spermatozoa can be performed using the morphology module of the CASMA system. We used the SCA (Sperm Class Analyzer®), version 6.5, equipped with a phase-contrast Eclipse Ci-S/Ci-L microscope (Nikon) and a Basler digital camera acA1300–200uc (Basler Vision Technologies) for automated morphology and morphometry analysis. Images of 200 sperm from each sample were captured, applying a 40X objective (Figure 3).
Keep in mind that the quality of the images captured and how well the system analyses the cells depends on your staining method and a properly set up microscope (Read blog entry Microscopy – part 2 by Gerhard van der Horst). Take your time and do this thoroughly; it will improve the quality of your results and avoid having to capture more sperm at a later stage.
Step 4: What is Normal? – Defining Your Own Breed-Specific Cut-off Values for Normal Sperm Morphology for Accurate Classification
While CASA systems come with default cut-off values for normal sperm morphology for each species, these often need to be adjusted for specific species or breeds. So, the question remains: what exactly is normal bull sperm and how do we define it for a Norwegian Red breed?
I chose to follow the so-called percentile method of Van der Horst et al. (2018) (Figure 4). We grouped sperm morphometric measurements of mature Norwegian Red bulls into a range of percentiles and used minimum and maximum values as the normal range of cut-off points for bull sperm in the CASMA system. We additionally validated our normal settings against specialists in the field in an online survey (morphology quiz). From this validation, we established our definition of normal, which was used for assessing all the samples from the young Norwegian Red bulls. After capturing all the images from all the samples, we performed a quality control of the images (verify the automated analysis) and deleted ones with incomplete masks.
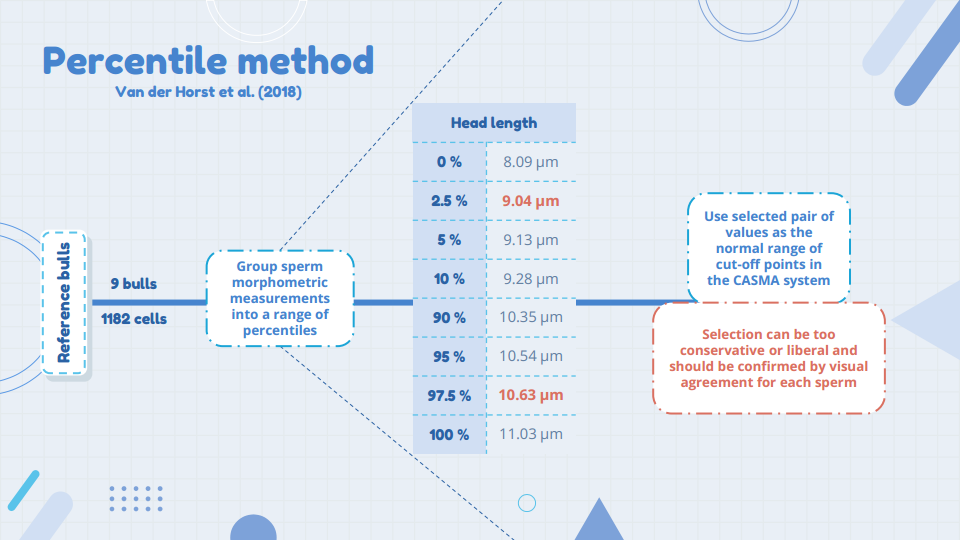
The sperm abnormalities were grouped and separately assessed. There are two type of morphology abnormalities which are not associated with infertility in the bull – distal droplets and abaxial tails. Due to the young age of the bulls, we decided to make a subjective assessment and count the distal droplets, as we expected them to be more prevalent. Accordingly, these and other tail abnormalities were added manually.
Conclusion
Following these four steps will optimise the morphology analysis for the particular samples analysed. The Computer-Aided Sperm Morphology Analysis (CASMA) system offers significant advantages in terms of objectivity and standardisation. However, it’s crucial to recognise that sperm morphology varies across species and even breeds. Therefore, careful calibration and adjustment of the CASMA system’s settings are essential to achieve accurate and reliable results for each specific use context. What we have done provided us with a balance between automation and expert input, allowing for more consistent and reproducible analyses while still accounting for the specie specific nuances and research objectives.
If you are interested in the results of our analysis, read the article “Novel interpretation of sperm stress test and morphology for maturity assessment of young Norwegian Red bulls” (https://www.sciencedirect.com/science/article/pii/S0378432023000751).
Joanna Bremer (PhD)
Researcher
Inland Norway University of Applied Sciences
Recommended reading list:
- Perry VEA. The Role of Sperm Morphology Standards in the Laboratory Assessment of Bull Fertility in Australia. Front Vet Sci. 2021 May 26;8:672058. doi: 10.3389/fvets.2021.672058. PMID: 34124227; PMCID: PMC8187580.
- Nöthling JO, Irons PC. A simple multidimensional system for the recording and interpretation of sperm morphology in bulls. Theriogenology. 2008 Mar 15;69(5):603-11. doi: 10.1016/j.theriogenology.2007.11.007. Epub 2008 Feb 1. PMID: 18242677.
- van der Horst G, Skosana B, Legendre A, Oyeyipo P, du Plessis SS. Cut-off values for normal sperm morphology and toxicology for automated analysis of rat sperm morphology and morphometry. Biotech Histochem. 2018;93(1):49-58. doi: 10.1080/10520295.2017.1380842. Epub 2018 Jan 10. PMID: 29319353.
- Bremer J, Heringstad B, Morrell JM, Kommisrud E. Novel interpretation of sperm stress test and morphology for maturity assessment of young Norwegian Red bulls. Anim Reprod Sci. 2023 Jun;253:107261. doi: 10.1016/j.anireprosci.2023.107261. Epub 2023 May 19. PMID: 37267747.
