The Cairo Consensus on Accreditation as the Basis for Future-Proofing the ART Laboratory
Our publication of the Cairo Consensus on accreditation as the basis for future-proofing the ART laboratory [Reproductive Biomedicine Online 2024:1041106; doi.org/10.1016/j.rbmo.2024.104106] is the latest in a series of international consensus reports aimed at encouraging best practices in the ART laboratory. It is the third ART laboratory consensus supported by the Upper Egypt Assisted Reproduction Symposium (UEARS) organization,* this time also in collaboration with Alpha Scientists in Reproductive Medicine and co-convened with Dr Mina Alikani.

The UEARS organization, headed by Dr Mohamed Fawzy, also supports consensus meetings in the areas of andrology and research integrity, all published in high profile peer-reviewed journals. It is important to note that the Cairo Consensus ART laboratory series is an evolution of, and in many ways built upon, the series of four Alpha consensus meetings that I planned between 2010 and 2016 (two of which were in cooperation with ESHRE’s SIG Embryology).**
The idea for a ‘future-proofing’ consensus meeting came to me during the COVID era when reading guidelines published by various professional groups on how laboratories around the world were coping with providing ART lab services during the pandemic. Those papers reflected different geographic and social perspectives, and I realized that while in some regions guidelines were often consistent with existing standard practices, in others they required the implementation of major changes. Hence adaptation to the pandemic conditions was more difficult in some parts of the world, or in some centres, than in others. But what struck me most was how many of the ‘new ways of working’ being recommended were needed in countries where specialized ART laboratory accreditation was not a major feature of practice, as behaviours would likely have already been in place had a formal accreditation scheme under-pinned their operations. This is not to question the competency of ART laboratories in those places, it’s just that they had not benefitted from operating under the total quality management (TQM) philosophy inherent to accreditation, especially its proactive emergency management and disaster planning aspects.
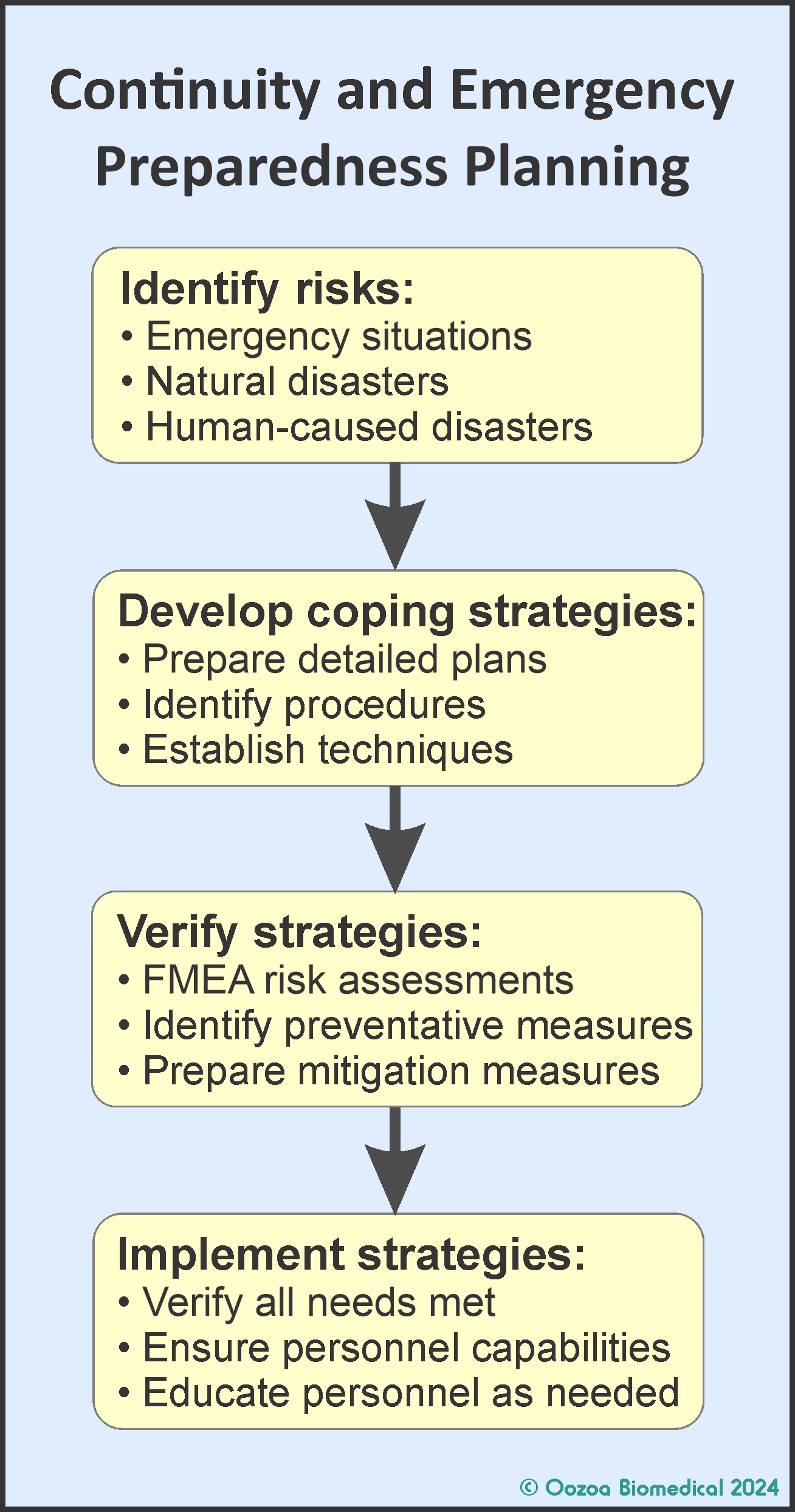
‘Future proofing’ refers to proactive planning and developing preparedness protocols to both withstand potential disruptions and safeguard the continuity of services. Its many facets include measures to enhance infrastructure, incorporate redundant capacities, optimize network coordination, and develop robust continuity of operations procedures, all aimed at mitigating the likely impacts of events such as natural disasters, other emergencies, and pandemics.
Accreditation is ‘a collegial process based on self- and peer-assessment whereby an authoritative body (usually a non-government organization) gives formal recognition that an organization is in voluntary compliance with one or more Standards set by the authoritative body’.*** It is different from both certification and licensing, which relate to following rules or obeying regulations. A comprehensive practice of proactive risk assessment is fundamental to all accreditation schemes.
Although accreditation should encompass the entire ART centre, we chose to focus the 2023 consensus on the ART laboratory, leaving patient management, clinical procedures, and public health matters, to appropriate other authorities. Our perspective was that, despite all the benefits of major accreditation schemes, it should not be necessary for an ART clinic to undergo formal accreditation (often at substantial cost) for the ART laboratory to be able to reap all those benefits. Any ART laboratory, anywhere, can focus on comprehensively employing the underlying TQM practices.
The Cairo Consensus group concluded that an international working group, comprising experienced representatives from all relevant stakeholder groups, should develop a globally applicable customized framework for accrediting ART laboratories. We envisaged a comprehensive technical accreditation guide for ART laboratories that could serve as a worldwide reference for ART laboratories seeking accreditation by any organization. It would also be a foundation document for accreditation bodies to harmonize their accreditation schemes and make them better suited to the specialized needs of ART laboratories, rather than just medical laboratories in general.
This will be a huge undertaking – but one with immeasurable benefits, not just to ART laboratory practitioners, but also to those who should (and must) be our primary concern: all the people we are helping to achieve their goal of creating a family.
Future Cairo Consensus meetings will continue to address key areas in optimizing ART laboratory practices, and we hope to expand our cooperation with Alpha in these endeavours. So “watch this space”!
Before closing, I must express my deepest gratitude to two people without whom the Cairo consensus meetings could not have happened. Firstly, Dr Mohamed Fawzy for his personal drive and support of the meetings, and secondly Dr Sharon Mortimer for all her support and help, both personal and professional. All those of us who have participated in one of the Alpha or Cairo consensus meetings are in awe of her ‘super-power’ of being able to condense a multi-stream, often quite intense, discussion among passionate experts into a simple concise statement that quickly becomes a lucid consensus point.
David Mortimer, PhD
President, Oozoa Biomedical Inc, Vancouver, Canada
CFAS Certified ART Laboratory Scientific Director
Member, Canadian Standards Association and Standards Council of Canada
Honorary Professor, School of Medicine, University of Dundee, Scotland, UK
Scientific Advisory Board member, Hamilton Thorne Ltd

References:
*Previous Cairo ART laboratory consensus meetings:
- Cairo consensus on the IVF laboratory environment and air quality: report of an expert meeting. UEARS Cairo Consensus Group; Cairo (Egypt), February 2017; co-convenors Dr David Mortimer and Dr Jacques Cohen. Published in Reproductive Biomedicine Online 36: 658-674, 2018, doi.org/10.1016/j.rbmo.2018.02.005.
- There is only one thing that is truly important in an IVF lab: everything. Cairo Consensus Guidelines on IVF Culture Conditions. UEARS Cairo 2018 Consensus Group; Cairo (Egypt), February 2018; co-convenors Dr David Mortimer and Dr Jacques Cohen. Published in Reproductive Biomedicine Online, 40: 33-59, 2020.
** Alpha consensus meetings:
- Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. ALPHA Scientists In Reproductive Medicine and ESHRE Special Interest Group Embryology; Istanbul (Turkey), February 2010; convenor Dr David Mortimer. Published in Reproductive Biomedicine Online 22: 632-646, 2011 and Human Reproduction 26: 1270-1283, 2011.
- The Alpha consensus meeting on cryopreservation key performance indicators and benchmarks: proceedings of an expert meeting. Alpha Scientists In Reproductive Medicine; Istanbul (Turkey), November 2011; convenor Dr David Mortimer. Published in Reproductive Biomedicine Online 25: 146-167, 2012.
- The Alpha Consensus Meeting on the professional status of the clinical embryologist: proceedings of an expert meeting. Alpha Scientists in Reproductive Medicine; Antalya (Turkey), May 2014; convenor Dr David Mortimer. Published in Reproductive Biomedicine Online 30: 451-461, 2015.
- The Vienna consensus: Report of an expert meeting on the development of ART laboratory performance indicators. ESHRE Special Interest Group of Embryology and Alpha Scientists in Reproductive Medicine; Vienna (Austria), September 2016; co-convenors Dr David Mortimer and Dr Giovanni Coticchio. Published in Reproductive Biomedicine Online 35: 494-510, 2017 and Human Reproduction Open 2017:hox011, doi.org/10.1093/hropen/hox011.
*** Mortimer ST, Mortimer D. Quality and Risk Management in the IVF Laboratory, 2nd edn. Cambridge University Press, Cambridge (UK), 2015.